The following is a repost of an article written by Mike DiCicco which can originally be seen on NASA’s site here.
In mid-March, as much of the country shut down in response to the rapidly spreading novel coronavirus (COVID-19), a team of engineers at NASA’s Jet Propulsion Laboratory in Southern California got to work.
Doctors nearby needed ventilators, so the team set out to design an inexpensive version that wouldn’t use any of the same parts as traditional ventilators, so as not to compete for supplies.
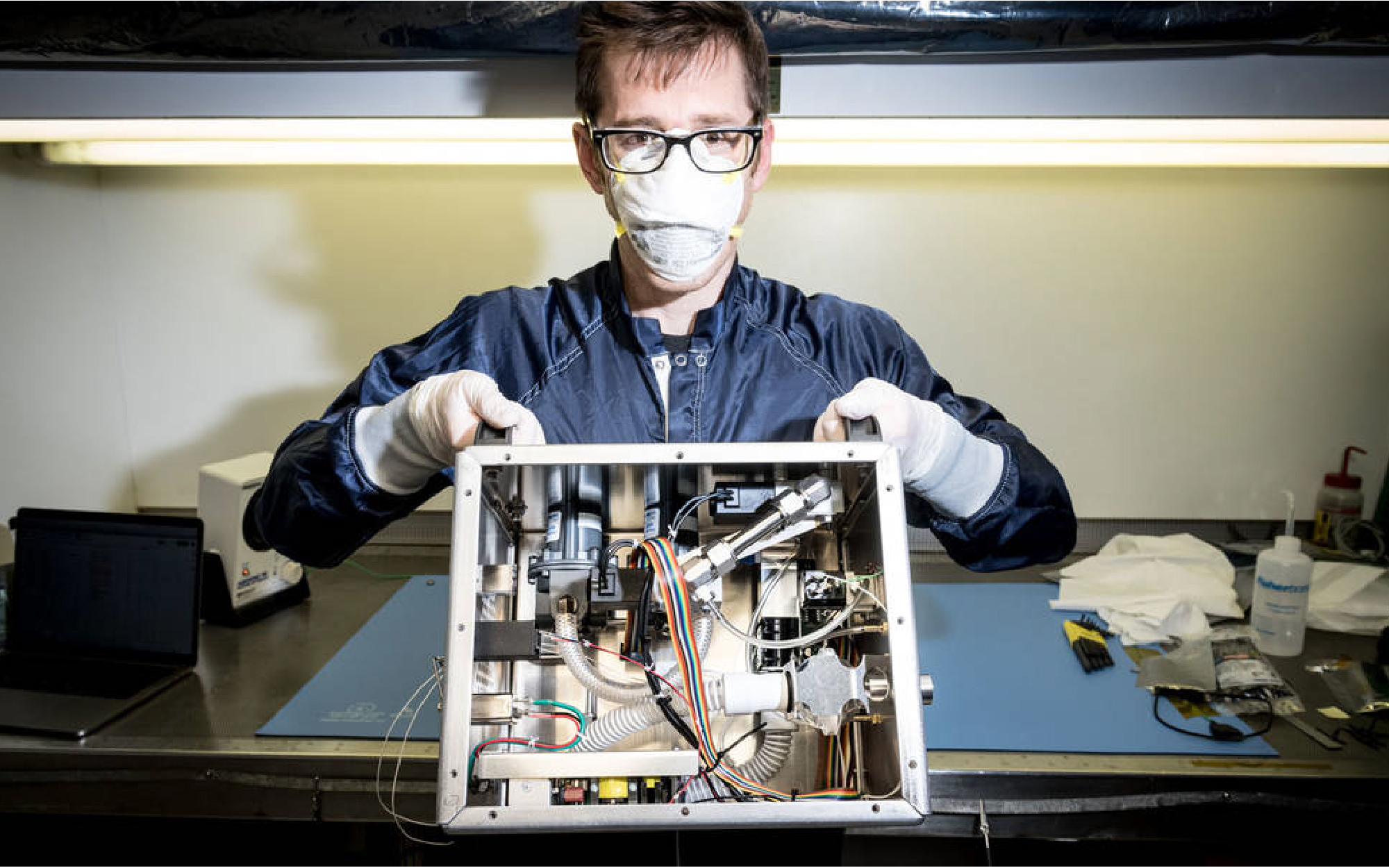
Patrick Degrosse, engineer at NASA’s Jet Propulsion Laboratory in Southern California, shows the guts of the ventilator that a team of NASA engineers designed in just over five weeks. The machine uses none of the parts used in traditional ventilators, so as not to compete for supply lines. Credits: NASA
Unsure where to begin and knowing that whatever they came up with would need rapid approval, they reached out to the Food and Drug Administration (FDA). Leon Alkalai, head of strategic partnerships for JPL, connected with the regulator’s assistant director in charge of respiratory devices. “I said, ‘We have no idea what we’re doing, but we have a great team and we’re enthusiastic and we need help,’” Alkalai recalled, “and he said, ‘We’re in.’”
The FDA official noted that ventilator design is essentially “a physics and fluidic problem,” Alkalai said. That was when he knew the team would succeed. “When the problem is translated to physics, we know what to do.”
Across NASA, other centers also found ways to refocus their skills and technologies to address the pandemic. As rates of infection and hospitalization again tick upward in many states, several of the solutions NASA field centers came up with in the spring now teeter on the verge of widespread application.
At NASA’s Johnson Space Center in Houston, home of the Human Health and Performance Center, the Technology Transfer Office combed through more than 2,000 technologies and software programs created in the last decade, looking for anything that might be useful in confronting the health crisis. The center submitted a portfolio of 34 open source technologies to the United Nations and is also helping a handful of groups update and manufacture a simple, human-powered ventilator originally designed for the space program.
Meanwhile, NASA’s Armstrong Flight Research Center in Edwards, California, joined a local public-private task force with a hospital and college, a neighboring city, and two spaceflight companies and ended up patenting an improvement to an oxygen helmet for COVID-19 patients.
And when NASA’s Glenn Research Center in Cleveland heard that a familiar company was working to update a device for sterilizing medical equipment and spaces, the center jumped in to help.
In all these cases, NASA and its partners found that, with a little guidance, aerospace engineers also make pretty good medical engineers.
If It Helps Save One Life
For JPL, quick turnaround of a viable emergency ventilator meant reaching out to many partners, said Alkalai, who initiated and managed all these relationships. These included two local hospitals, several federal agencies, the University of California Los Angeles, and medical device giant Medtronic.
After just 37 days of working around the clock, they had a prototype, called Ventilator Intervention Technology Accessible Locally, or VITAL for short. “There were issues of exhaustion, but we were on a mission,” Alkalai said.
Almost as quickly, the FDA granted the device a ventilator emergency use authorization. The next trick was to get it out into the world. This required a new approach to licensing.
“Normally, we’re happy if just one company comes to us saying they’re interested in a license,” said Daniel Broderick, manager of JPL’s Technology Transfer Office. In this case, the response was much bigger. Over 300 companies registered on the JPL website to learn more about the ventilator, and more than 100 applied for a license. Now the challenge was to determine who was capable of producing the machine. “We’ve never seen this much licensing demand for a technology,” Broderick said.
One of those applicants was Pro-Dex Inc., a design and manufacturing company in Irvine, California. Working with NASA on the ventilator was an opportunity to learn new things, grow the company, and “be part of the solution,” said Pro-Dex CEO Rick Van Kirk.
In late June, the company was working on sourcing parts, determining distribution channels, and laying out the assembly line. And NASA is still supporting the effort, having put together documentation, 3D renderings, and videos to assist licensees, including a video about the assembly process. “They did a great job of teeing it up for everybody,” said Van Kirk.
Pro-Dex was one of 29 companies granted licenses, including seven other U.S. businesses.
“If half of them end up delivering the devices, that would be amazing,” said Alkalai. “We would be just thrilled if at least one unit makes it into a hospital and helps save a life.”
Other teams at JPL have designed protective respirator masks and a necklace that vibrates when wearers start to touch their faces. The masks and necklace can be 3D printed, and the design files and instructions are available for open source licensing on GitHub.

About 30 entities have licensed the low-cost Ventilator Intervention Technology Accessible Locally, or VITAL, that NASA engineers designed and patented. Licenses are free of charge. Credits: NASA
Human-Powered Solutions
Engineers at Johnson are offering a simpler ventilator solution, primarily for use in developing countries. As the pandemic unfolded, engineers who had developed a ventilator for use on the Orion spacecraft started updating it. The device is similar to human-powered ventilator bags used in ambulances, but those are squeezed by hand, which becomes tiring quickly. Johnson’s ventilator is powered by larger muscle groups in the arms or even legs. It can be used to keep a patient alive for hours, perhaps while waiting for a bed to open up, said Kris Romig, technology transfer officer at Johnson.
“The technical team came to us and said, ‘We think this could help, and we don’t know how to get it out into the world,” he said. The center is now offering the ventilator as an open source technology.
It didn’t take long for Matthew Fiedler and the other founders of 3D printing company re:3D, all former Johnson employees, to hear about the ventilator, which the company is helping to refine.
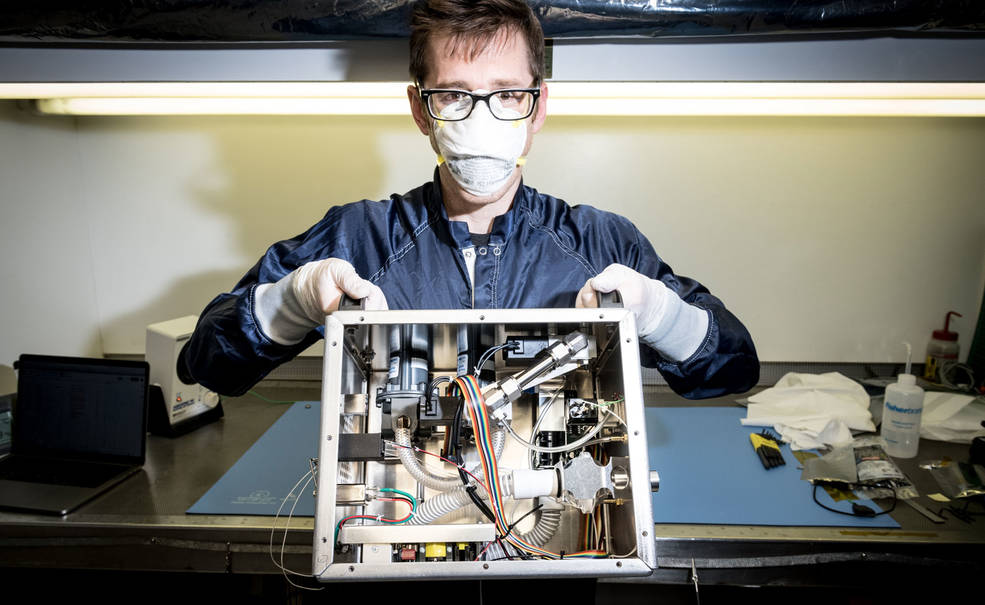
A team at NASA’s Johnson Space Center in Houston designed a 3D-printable ventilator that can be powered with both hands for use in the Orion capsule. The center has repurposed it for use on COVID-19 patients and is working with companies around the world to get it out to hospitals. Only a few parts, such as the accordion-like bellows, can’t be 3D printed. Credits: re:3D
The Johnson team had computer-aided design files for the ventilator parts but had never manufactured them. “They sent us the file, and we printed it,” Fiedler said. “We’re helping them bring the product to life and figure out how to make it better.”
Once the design is finalized, re:3D, whose manufacturing facility is close by Johnson in Houston, could start producing ventilators, working with federal and international organizations to get them into the hands of those who need them, he said.
Anheuser-Busch InBev (ABI), whose global technical headquarters is in St. Louis, is also working to get Johnson’s manual ventilator out into developing countries. “We deliver beer to places you wouldn’t believe all over the world,” said Lucas Steinle, global director of industrial digital transformation at ABI, noting the company could use that infrastructure to help deliver the ventilators almost anywhere.
The engineering group of ABI’s subsidiary in South America, known as Ambev, is working with Johnson engineers to finalize a prototype, which it plans to bring to the United Nations to see how the company can partner with other groups to get it into manufacturing and distribution. Steinle added that ABI has the facilities to manufacture it through 3D printing if need be.
Meanwhile, Leviathan Space Industries is building partnerships to introduce the human-powered ventilator in Ecuador. The company has been working to build a private spaceport in the Ecuadorian city of Guayaquil, which was ravaged by one of the world’s worst outbreaks of the virus.
“Due to its ease of use and how cheap it is, this can definitely help provide emergency relief when hospitals have overflow capacity,” said Robert Aillon, founder of Leviathan.
The Pompano Beach, Florida-based company has partnered with the University of Kentucky for help with testing and FDA approval and is working with Ecuadorian company Pica Plasticos Industriales on manufacturing. And Leviathan is working with the Ecuadorian school Universidad Espiritu Santo to help with that country’s regulatory approval process, Aillon said.
Back at Johnson, the center’s simultaneous effort to dig up any technology that might help – whether or not it’s patented – has led the Technology Transfer Office to consider making it possible for the public to search broad categories of unpatented technology. “These can be useful without a license, just open source,” Romig said.
A Second-Generation Sterilizer
While others work on ways to mitigate the effects of the virus, the company Emergency Products and Research (EP+R) is working with Glenn engineers to destroy it.
The Kent, Ohio-based company’s AMBUstat fogger system creates an aerosol of water, peracetic acid, and hydrogen peroxide to eliminate all pathogens in the air or on surfaces. It was originally developed after consultation with a Glenn research engineer in 2015 and was intended for use in ambulances.
“We were working on a new design that would let us deal with the limitations of the original,” said Jason Thompson, who handles business development for EP+R and drove the original device’s creation. The company wanted it to better address airborne contaminants, treat different-sized spaces more efficiently, and be more cost-effective.

With help from NASA’s Glenn Research Center in Cleveland, the company Emergency Products and Research (EP+R) improved its AMBUstat sterilant. Jason Thompson of EP+R tests a new system that lets the AMBUstat G2 device quickly sterilize small spaces, like the inside of a police car. Credits: Emergency Products and Research
When Glenn heard about the new work, the center wanted to help again, so it put an aerosol science and instrumentation specialist on the case, and JPL was tapped for additional consulting. The resulting device, known as the AMBUstat G2, creates smaller aerosol droplets to better attack airborne viruses. Improved flow control and the ability to control the process from outside of the targeted space allow it to treat spaces faster and more effectively. In a pilot project with the Ohio State Highway Patrol, the company found it could disinfect 10 to 12 police cars in the time the original fogger treated just one.
Following about a month and a half of cooperation, Glenn is testing the new device, after which it will go to a proving ground for testing against the novel coronavirus.
With the sterilant already approved by the Environmental Protection Agency, Thompson said, the company is ready to move into production of the AMBUstat G2 as soon as testing is complete.
Meanwhile, the Glenn researcher who helped refine the original AMBUstat teamed up with researchers from University Hospitals Health System in Cleveland to develop another device that uses atomic oxygen to decontaminate N95 facemasks for reuse. Initial results indicate effectiveness; however, more testing is needed to confirm the effect of multiple decontamination cycles on the integrity of the masks.
Over at Armstrong, the Technology Transfer Office was hard at work pursuing FDA approval and a company to build an improved oxygen-supplying device the center’s engineers came up with.
The positive-pressure oxygen helmet resulted from a task force that included Armstrong, spaceflight company Virgin Galactic and its sister The Spaceship Company, the city of Lancaster, Antelope Valley Hospital, and Antelope Valley College, bringing together resources, medical professionals, and engineers.
“Completely Outside of Our Comfort Zone”
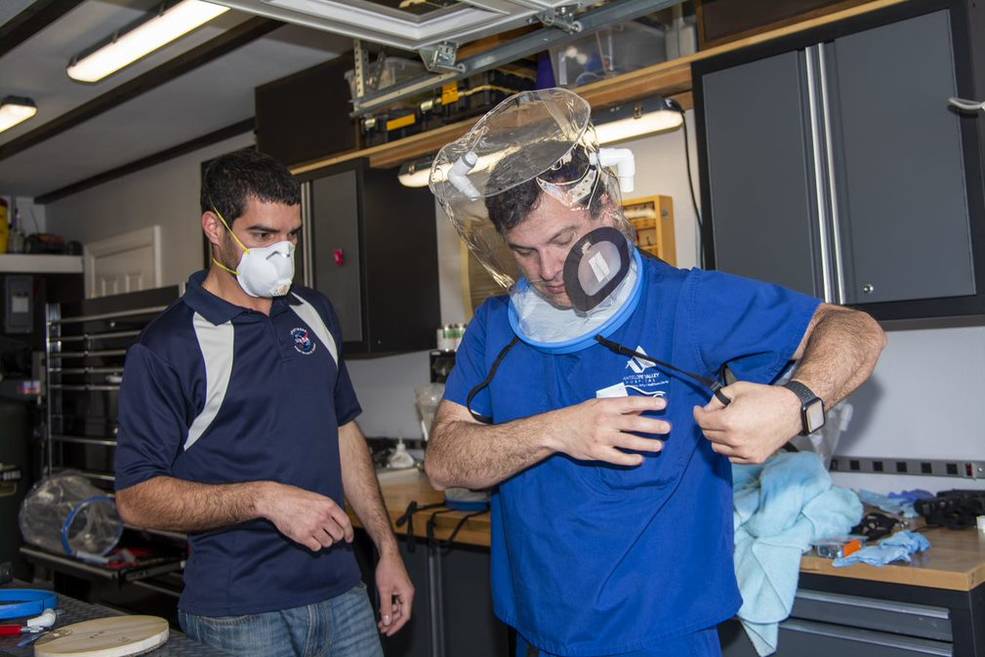
Engineer at NASA’s Armstrong Flight Research Center in Edwards, California, Mike Buttigieg (left) led a team that came up with a low-cost oxygen helmet for COVID-19 patients. The design includes a magnetically sealed port, which the center has licensed out. Here, Dr. Daniel Khodabakhsh of Antelope Valley Hospital tries one on. The hospital was part of a task force that helped with the effort. Credits: NASA
Oxygen helmet manufacturers have been unable to meet the surge in demand in response to COVID-19, which often deprives patients of oxygen. A team led by Armstrong engineer Mike Buttigieg was charged with developing a low-cost, easily made assisted breathing helmet that could withstand pressures that off-the-shelf units weren’t designed for, without impacting the supply chain. Through conversations with the team’s lead doctor, Buttigieg had the idea to install a magnetic port, allowing access to the wearer’s face. “Having a helmet on without face access makes it hard to check vitals or take a drink of water,” said Samantha Hull, licensing manager and outreach coordinator at Armstrong.
The task force produced hundreds of the modified helmets for use at local hospitals, but Armstrong wanted to get them produced at greater scale. Final FDA approval also required a commercial manufacturer, meaning NASA had to find a company to license the technology without regulatory approval, said Benjamin Tomlinson, technology transfer officer at Armstrong.
In early July, the brand-new company Medify Products LLC signed a nonexclusive license to use the magnetic access port in oxygen helmets.
Tom Ryder, president and CEO of Genesis Plastics Welding, started Medify Products after he saw video of oxygen helmets being used in Italian hospitals early in the crisis. Genesis, his original company, had been producing similar helmets for more than 25 years.
“This is a product that utilizes all of our expertise,” he said. “We want to put that talent to use in fighting the virus.”
Ryder said Medify, located in Fortville, Indiana, will likely incorporate Armstrong’s magnetic port into more than one helmet design. A major advantage of working with NASA, he said, is that Armstrong is working with its contacts to get prototypes into formal testing and working with the FDA to secure emergency authorization for the helmets.
Much of this is new territory for Armstrong, which specializes in aeronautical research. “Medical applications are completely outside of our comfort zone,” said Tomlinson, noting that his team is figuring out how to navigate the approval process.
“This is something you can produce without a lot of expense, and it can save lives,” said Tomlinson. “Its elegance and simplicity is the beauty of it.”
Ryder said he wouldn’t previously have associated NASA with projects like this. “How they’re working with businesses like mine, a small business, gives me hope for the country.”
To learn more about NASA’s response to coronavirus, visit: https://medeng.jpl.nasa.gov/covid-19/
Mike DiCicco
Article Author